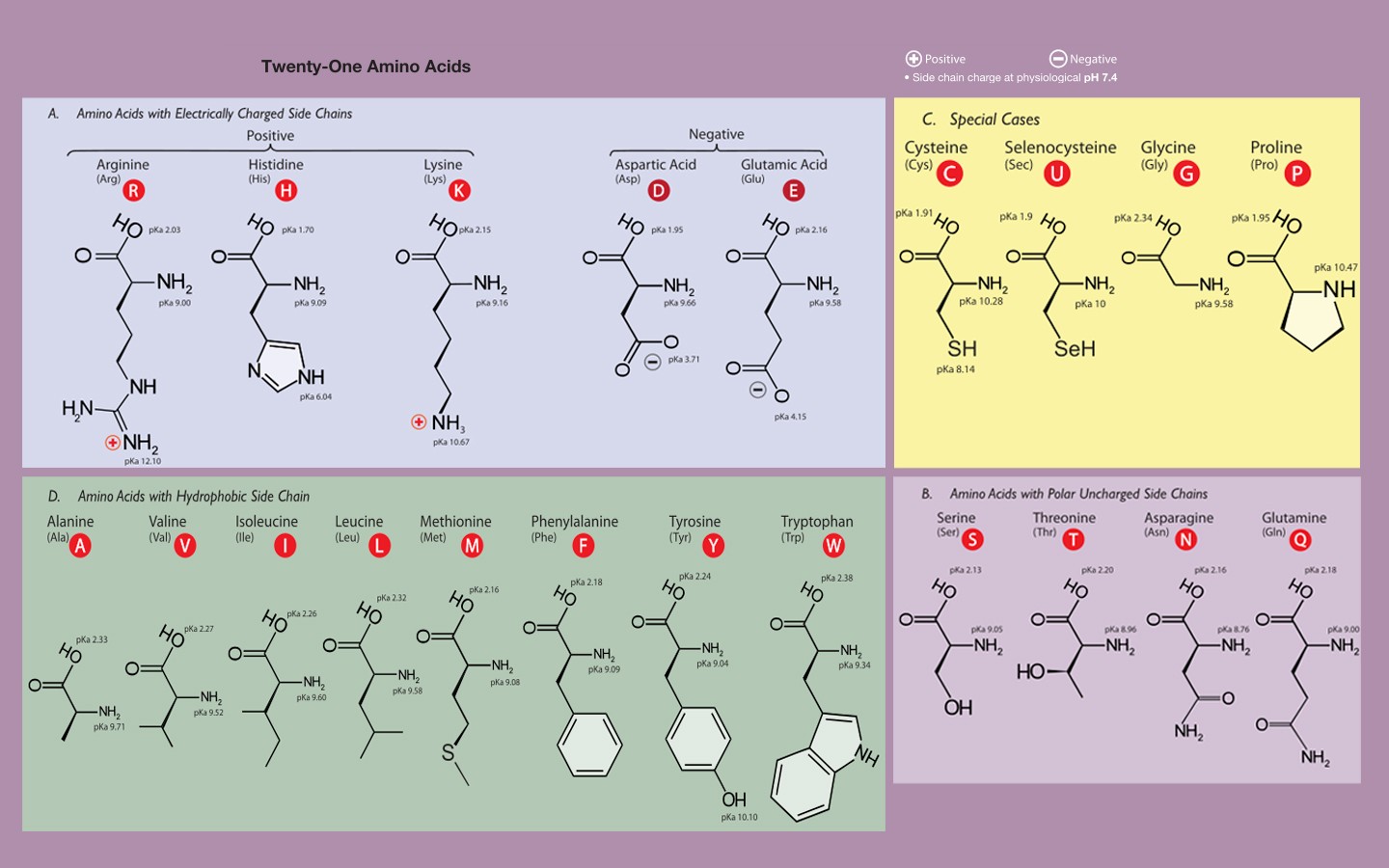
Amino acids are small organic acids containing an amine (-NH2) group and a carboxylic acid group (-COOH). In solution these two terminal groups ionize to form a doubly-ionized, though overall neutral entity called a zwitterion. The amine donates an electron to the carboxy group and the ionic ends are stabilized in aqueous solution by polar water molecules.
Amino acids are the basic building blocks of proteins, which are long chains or polymers of amino acids. In living systems, the instructions which code for the cellular synthesis of a specific protein are contained in nucleic acids (DNA); the process of protein synthesis from the genetic information is termed expression.
Twenty or so amino acids are termed essential because the body cannot synthesize them from other amino acids. Bulk proteins, as in animal muscle tissue (meat), contain an abundance of all varieties of amino acids, which are liberated in the stomach by cleavage by proteolytic enzyme. Some amino acid oligomers pass the lacteal boundary and enter the bloodstream, where they constitute a toxic threat as proteins not endemic to the organism.
Phenylalanine and tyrosine are the two major precursors to dopamine, epinephrine (adrenaline) and norepinephrine, the body’s three “fight or flight” hormones. Tryptophan is the main precursor to serotonin, an important inhibitory neurotransmitter controlling the stimulative adrenalin group compounds. Tryptophan is also used by the body to synthesize vitamin B3 when insufficient B3 is derived from diet (or from the symbiotic flora present in the GI tract). Glutamine is precursor to glutamate and GABA, two central neurotransmitters. Glutamate and aspartate also function as neurotransmitters in the brain. In addition, glutamate combines with cysteine and glycine ans the mineral selenium to form a tripeptide free-radical chelating agent called glutathione. Glutamine is also burned by the brain as a fuel, and basically imparts a feeling of energy. GABA is an ubiquitous inhibitory amino acid, both centrally and peripherally.
Leucine is used by the body to regulate blood sugar levels; one can increase one’s blood sugar by consuming isoleucine, which competes for absorption with leucine, reducing blood leucine levels. (All but the simplest molecules compete for absorption across the lacteals in the intestine.) Leucine, isoleucine and valine are called branched-chain amino acids; they are metabolized in muscle tissue and are important for muscle synthesis. Proline, a non-essential amino acid, is a major constituent of collagen.
Serine and threonine contain alcohol (-OH) groups, which can be used as active groups in enzymes, as can sulfhydryl (-SH) containing aminos. Methionine and cysteine constitute the major sulfur-containing amino acids; they are important in maintaining elasticity in peptide macromolecules like hair, skin, and fingernails, since bonds between protein strands are typically disulfide bonds. Methionine is a rich constituent of meats, but is lacking in most vegetables. Lysine is another amino acid important to collagen formation, bone growth, etc., which can be lacking in vegetarian diets. Methionine is also important for metabolizing toxins like alcohol and acetaminophen, for example. Along with cysteine, it has the ability to chelate heavy metals.
Histidine is the precursor of histamine, which is the body’s messenger of allergic response. Consuming more histidine, rather than making one more susceptible to allergic reaction, may help decrease the number of helper cells, reducing allergy symptoms. Arginine is important in immune function, sperm production, and wound healing, and may reduce blood cholesterol levels.
Carnitine is one of the so-called catabolic amino acids; it aids in transporting fat for oxidation (lipolysis). Taurine is another catabolic amino acid which probably performs inhibitory functions in the CNS. It is “conditionally essential,” important mainly during development. Taurine levels are characteristically low in brain tissues following epileptic seizures. Aspartate and methionine also perform catabolic functions. These are important when losing weight, or when changing health-related habits such as smoking or alcohol consumption, since the body must tear down or reconstitute old structures as well as building new ones (anabolics). Carnosine is a dipeptide constituent of muscle tissue, as its name implies. Calanine is a putative anticancer agent from legumes which forms covalent oximes with vitamin B6-cotaining enzymes. Canavanine is a similar aminooxy compound with enzyme-inhibiting properties, foundin legumes and alfalfa.
Cysteamine is a sulfhydryl compound used as an antidote to acetaminophen poisoning. Kyurenine and kyureninic acid are produced in body from tryptophan; this production is stepped up in B-vitamin avitaminoses. Djenkolic acid occurs in the djenkol bean; it is structurally similar to cystine, the dimeric form of cysteine. Hypaphorine is a convulsive poison similar to tryptophan.
proteins & chemical evolution
Proteins are one of the main products of DNA. Genetic codes in chromosomes allow cells to generate or express specific proteins using amino acids as building blocks. Through this means, DNA builds cellular machinery and controls its environment. The famous Miller experiment, conducted some decades ago, simulated conditions on the early earth in a beaker; an electric spark in an atmosphere of water, oxygen, CO2 and ammonia, produced an abundance of amino acids.
Looking over this chemical alphabet, one sees that they are the simplest combinations of the most common, most reactive elements present on early earth. Nitrogen is the most abundant, most reactive electron donor while oxygen is the most abundant, most reactive electron receptor. Carbon, meanwhile, is like a bisexual chemical slut – it can swing either way, and it bonds with just about anything. In short, the building blocks of life turn out to be just what you would expect, the simplest possible combinations of light elements. This bodes well for the theory of natural selection, extended to the chemical realm.
mad cow disease
Interestingly, prions, the infectious proteins responsible for “mad cow disease” (bovine spongiform encephalopathy), are non-DNA entities (unlike viruses and bacteria). Fragments of harmless proteins, these “rogue” proteins assume a new conformation and alter existing neuronal proteins, causing tissue damage. This is a radically alien way of conducting chemical commerce, as one might find on another planet or galaxy, as in that old Star Trek episode with the giant extragalactic space amoeba!